Abstract
Introduction The challenges posed by the increasing incidence of hematologic malignancies in older patients are significant and while during the past 20 years there has been a dramatic expansion in the therapeutic armamentarium against hematologic malignancies, there is a need for data to guide treatment decisions for older adults, particularly with respect to the use of novel targeted or immunotherapeutic agents. To a large extent, this is a consequence of the under-representation of this growing demographic in cancer-related clinical trials.
Management of primary CNS lymphoma (PCNSL) in patients age ≥ 70 years represents a significant problem. While it is established that older PCNSL patients benefit from high-dose methotrexate-based induction regimens, whole brain irradiation consolidation is not a favored option because of excessive neurotoxicity. While there is evidence that high‐dose chemotherapy improves outcomes in patients age < 70, dose‐intensive chemotherapy is not an option for most older PCNSL patients. Given that the median age of PCNSL at diagnosis is ~ 60 years, determination of the optimal consolidative approach for older patients is an important question. This problem is particularly significant given that the incidence of PCNSL continues to rise in this older age group.
While PCNSL increasingly appears to be a curable brain tumor, outcomes for patients age > 60 remain poor, with 1-year progression-free survival (PFS) of ~ 40% and median overall survival of 14-30 months. Population-based registry data suggests a median overall survival for PCNSL patients age > 70 of approximately 7 months over the past decade. New therapeutic approaches are needed for the particularly vulnerable older PCNSL patient population.
Given the evidence for activity of low-dose lenalidomide in relapsed primary and secondary CNS lymphoma, in 2011, we began using low-dose lenalidomide as maintenance in consecutive older PCNSL patients (age ≥ 70 years, HIV negative) following standard methotrexate/rituximab-based induction, in lieu of surveillance, whole brain irradiation or high-dose chemotherapeutic consolidation. Here we report on the characteristics, outcomes, toxicities, progression-free and overall survival of the first 11 PCNSL patients, age ≥ 70, to receive lenalidomide maintenance after high-dose methotrexate-based induction at our institution.
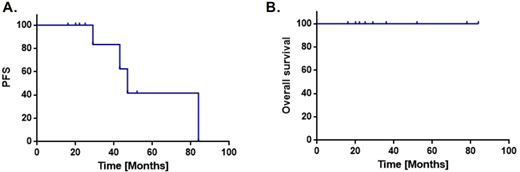
Results Eleven patients age > 70 received low-dose lenalidomide maintenance 5-10 mg/d on a 21-d cycle after induction therapy for PCNSL.Median age of the cohort is 77 years (range 70-86). Median Karnofsky performance status (KPS) is 60 (range 50-80); median IELSG prognostic risk score is 4 (range 3-5). The median methotrexate dose administered was 2.5 grams/m2 (range 0.5 - 8). With overall median follow-up of 30 months, median time on lenalidomide maintenance is 14.8 months (range 0.9 - 65.2). Two patients received lenalidomide plus maintenance rituximab, every 6 months. Median PFS is 48 months (range 16.8 - 84.8). Median OS has not been reached, and there have been no deaths (Figure 1). Four patients experienced disease progression on lenalidomide; 3 of them continued maintenance lenalidomide after salvage and 1 received maintenance pomalidomide. Lenalidomide maintenance has generally been well-tolerated. However, 5 toxicities potentially related to therapy have mandated cessation of lenalidomide: 1 grade 2 arthralgia, 1 subdural hematoma, 1 pneumonia, 1 grade 3 fatigue, 1 deep vein thrombosis.
Conclusions These encouraging preliminary results of prolonged PFS and OS with low-dose lenalidomide illustrate the feasibility and challenges, as well as suggest a potential benefit of maintenance therapy with low-dose lenalidomide in older patients with PCNSL. Nine of 11 patients were able to complete at least 8 months of lenalidomide maintenance after induction therapy. Toxicities were manageable and strikingly, there have been no deaths. Recently, lenalidomide has been shown to enhance the proliferation, survival, and chemotactic responses in T-cells isolated from older subjects. This suggests a potential mechanistic basis by which low-dose lenalidomide may improve immunity in the elderly and potentiate response to chemotherapy. Future studies are needed to prospectively test the benefit and mechanisms of lenalidomide maintenance in older patients with PCNSL.
Rubenstein:Genentech: Research Funding; Celgene: Research Funding. Mannis:AbbVie: Membership on an entity's Board of Directors or advisory committees; Agios: Research Funding; NKarta: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal